Pneumococcal Vaccine
Participate in clinical trials for the Pneumococcal Vaccine to support groundbreaking research focused on improving prevention and care. Your involvement helps develop innovative solutions to protect against pneumococcal diseases, enhancing outcomes and public health. Join us today and make a meaningful impact!
Showing all 2 results
-
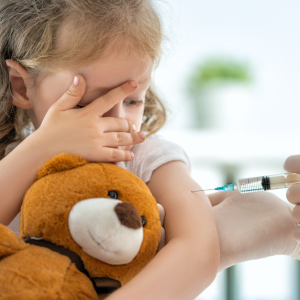
Pneumococcal Vaccine
We’re conducting a study to evaluate vaccines for infants to enhance their protection against common illnesses. Your baby's participation could contribute to advancing pediatric care while receiving routine vaccinations. Criteria & Qualifications: • Infants aged 2 to 3 months. • Healthy and medically stable. • Born full-term or preterm but meeting weight requirements. Compensation: Contact us for more information
Pneumococcal Vaccine
Read more -

Pneumococcal Vaccine Study
Support groundbreaking research by participating in our Pneumococcal Vaccine Study. Your involvement could help improve vaccine protection for children everywhere. Criteria & Qualifications: • Your child is eligible if they meet the study's age requirements. • Able to commit to 7–8 study visits over a period of 16–19 months. • Parent or guardian must be able to attend all 7–8 study visits over 16–19 months. Compensation: Contact us for more information.
Pneumococcal Vaccine Study
Read more
