Clinical Trials, phases I-IV, and types of Randomized Trials.
Clinical trials are research studies performed in people that are aimed at evaluating a medical, surgical, or behavioral intervention. They are the primary way that researchers find out if a new treatment, like a new drug or diet or medical device (for example, a pacemaker) is safe and effective in people. Often a clinical trial is used to learn if a new treatment is more effective and/or has less harmful side effects than the standard treatment. Randomized Clinical Trials
Ra
Clinical Trials and Study Types:
There are two main types of clinical trials or studies – interventional and observational.
Interventional trials aim to find out more about a particular intervention, or treatment. People taking part are put into different treatment groups so that the clinical research team can compare the results.
Observational studies aim to find out what happens to people in different situations. The clinical research team observes the people taking part, but they don’t influence what treatments people have. The people taking part aren’t put into treatment groups.
There are different types of clinical trials within these two groups. This page has information about
- Pilot studies and feasibility studies
- Prevention trials
- Screening trials
- Treatment trials
- Multi-arm multi-stage (MAMS) trials
- Cohort studies
- Case-control studies
- Cross-sectional studies
What Are The Trial Phases in a Clinical Study?
- Clinical trials testing new treatments are divided into different stages, called phases. The earliest phase trials may look at whether a drug is safe or the side effects it causes. Later phase trials aim to test whether a new treatment is better than existing treatments.
- There are 3 main phases of clinical trials – phases 1 to 3. Phase 1 trials are the earliest phase trials and phase 3 are later phase trials.
- Some clinical trials have an earlier stage called phase 0, and there are some phase 4 trials done after a drug has been licensed.
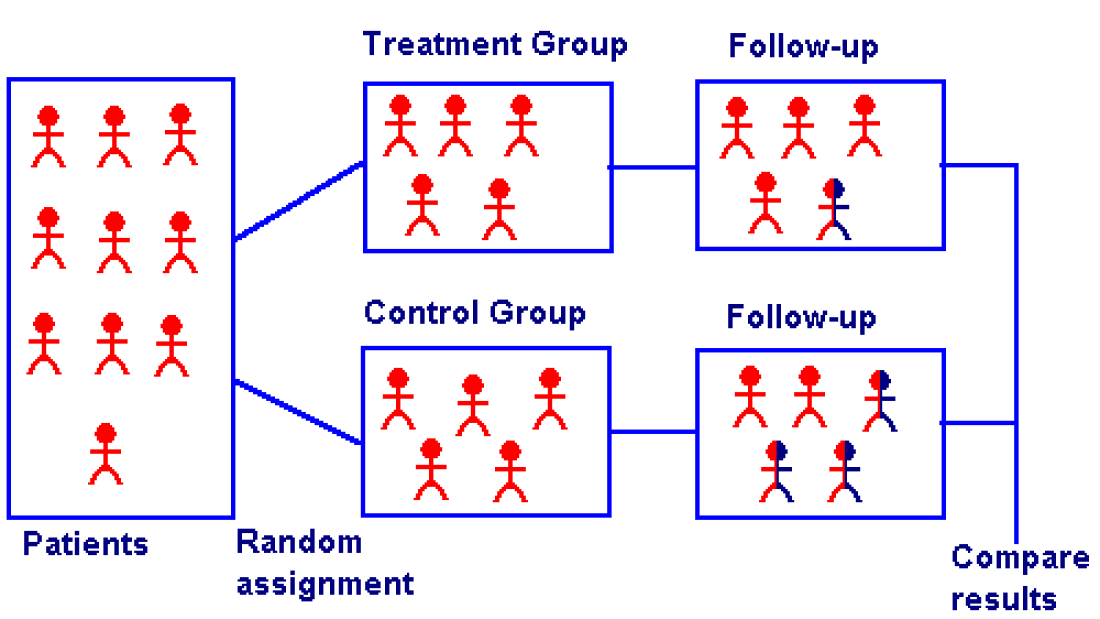
- Some trials are Randomized Clinical Trials. This means the people taking part are put into one of the treatment groups at random. Doing this means the results are more reliable.
Clinical Research: Trial Phases at a Glance
| Phase | Number of people taking part | Main aims of trial | Is it randomized? |
| 0 | Small – often about 10 to 20 people | Testing a low dose of the treatment to check it isn’t harmful | No |
1 | Small – often about 20 to 50 people | Finding out about side effects, and what happens to the treatment in the body | No |
2 | Finding out more about side effects and looking at how well the treatment works | Sometimes | |
| 3 | Large – hundreds or thousands of people | Comparing the new treatment to the standard treatment | Usually |
| 4 | Medium to large, variable | Finding out more about long term benefits and side effects | No |
Randomized Controlled Trials (RCTs)
A randomized controlled trial (RCT) is a way of doing impact evaluation in which the population receiving the programme or policy intervention is chosen at random from the eligible population, and a control group is also chosen at random from the same eligible population. It tests the extent to which specific, planned impacts are being achieved.
In an RCT, the programme or policy is viewed as an ‘intervention’ in which a treatment – the elements of the programme/policy being evaluated – is tested for how well it achieves its objectives, as measured by a predetermined set of indicators. The strength of an RCT is that it provides a very powerful response to questions of causality, helping evaluators and programme implementers to know that what is being achieved is as a result of the intervention and not anything else.
The simplest Randomized Clinical Trials (RCT) design has one treatment group (or ‘arm’) and a control group. Variations on the design are to have either:
– multiple treatment arms, for example, one treatment group receives intervention A, and a second treatment group receives intervention B, or
– a factorial design, in which a third treatment arm receives both interventions A and B.
Simple Randomized Clinical trials
In situations where an existing intervention is in use, it is more appropriate for the control group to continue to receive this, and for the RCT to show how well the new intervention compares to the existing one.
In a simple Randomized Clinical Trials (RCT), the unit of analysis for the intervention and for the random assignment is the same. For example, when evaluating a programme that provides nutrition to individuals, individuals might be randomly assigned to receive nutritional supplements.
For both practical and ethical reasons, however, it is more usual to use a cluster Randomized Clinical Trial’s design, in which the unit of assignment contains multiple treatment units. For example, education interventions are usually assigned at the school level, although the intervention takes place at the level of the teacher, classroom or individual child, and effects are measured at the level of the child. Nutrition interventions, for example, can be assigned at the community or sub-district level. Given the kinds of large-scale programmes supported.
Source: Unicef