Clinical Research would be a lot more difficult without the help of Clinical Research Organizations (CROs). These companies within the pharmaceutical industry not only help support drug companies but also companies in the biotechnology and medical device industries as well.
Some of the myriads of services CRO’s offer are, help with the development, testing, research, and commercialization of products within multiple fields. CRO’s are designed to reduce costs for companies developing new therapies and drugs in niche markets.
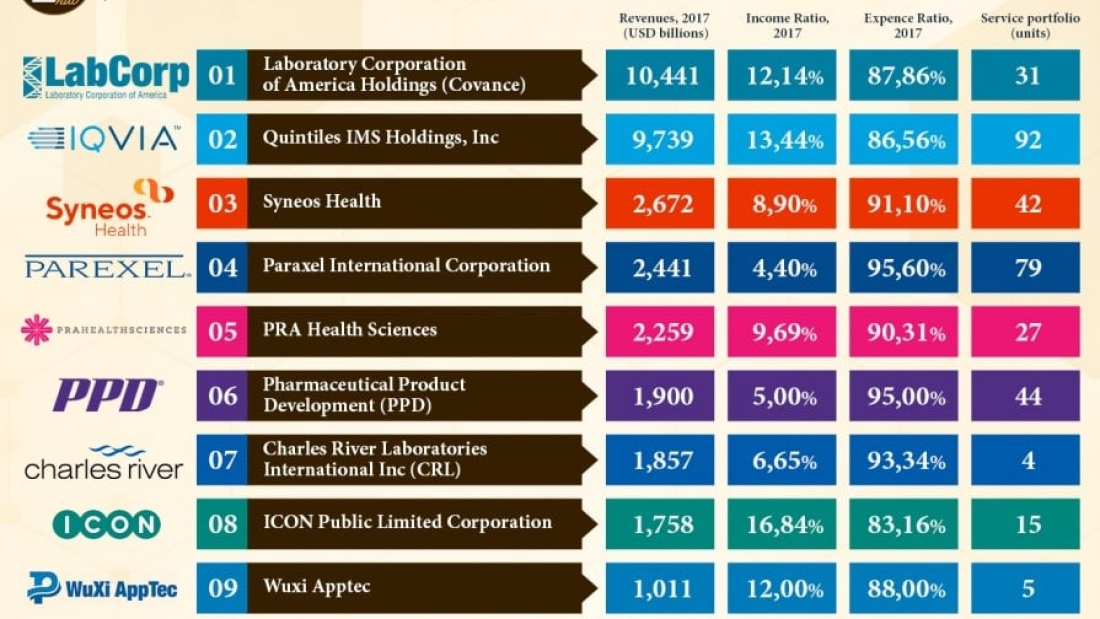
Listed below are the top CRO’s in the industry measured in terms of, total revenue, revenue per employee, growth between 2016-2017, net income, and expenses.
We shall now take a closer look at each of the CROs to see what qualities make them the best.
Covance, a global contract research organization and drug development services company, has helped bring 49 of the top 50 best-selling drugs to market. We’re dedicated to providing high-quality nonclinical, preclinical, clinical, and comercialización services to pharmaceutical and biotechnology companies to help reduce the time and costs associated with drug development.
Covance is headquartered in Burlington, NC, and ranked first among the top CROs across the globe owing to its revenue. The company recorded its revenues at $10.441billion, an increase of nearly 8.28% from the previous year. The Company reports its business in two segments, LabCorp Diagnostics (LCD) and Covance Drug Development (CDD). Covance Drug Development is a provider of end-to-end drug development services from early-stage research to regulatory approval and beyond. The company has a sophisticated laboratory network globally with more than 50,000 employees in 2017.
In October 2016, IMS Health, the dominant provider of pharmaceutical market data, merged with Quintiles to form QuintilesIMS, this merger is notable because Quintiles, the company acquired by IMS, was not a data company, but a contract research organization (CRO). QuintilesIMS announced that it would be changing its name to IQVIA, this distinctive merger reflects recent shifts in the paradigms for drug research, development, and marketing approval.
“We are committed to providing solutions that enable healthcare companies to innovate with confidence, maximize opportunities, and, ultimately, drive healthcare forward. We do this via breakthroughs in insights, technology, analytics, and human intelligence that bring the advances in data science together with the possibilities of human science.“ IQVIA announced the new company commitment.
IQVIA became the second-largest player in the ranking as it recorded revenues at USD9.739 billion, an increase of nearly 41.59% from the previous year. The Company reports its business in two segments, Commercial Solutions, and Research & Development Solutions
INC Research Holdings, Inc, a global Contract Research Organization CRO, an inVentiv Health, Inc a privately held global CRO and Contract Commercial Organization (“CCO”), announced their merger. “The only fully integrated biopharmaceutical solutions organization. Our company, including a Contract Research Organization (CRO) and Contract Commercial Organization (CCO), is purpose-built to accelerate customer performance to address modern market realities”. Syneos Health pronounces about their merge.
Currently, Syneos Health has more than 21,000 clinical and commercial minds that support customers in more than 110 countries. The company has recorded its revenues at USD2.672 billion, an increase of nearly 65.96% from the previous year.
PAREXEL International is a multinational, life sciences consulting firm and contract research organization (CRO), they are ranked fourth in the list with a revenue of$ 2.441 billion and have the second-largest service portfolio. The company provides a broad range of expertise in clinical research, clinical logistics, medical communications, consulting, commercialization, and advanced technology products and services to the pharmaceutical, biotechnology, and medical device industries. The company operates from 85 locations and has approximately 18,900 employees throughout 52 countries around the world.
PRA Health Sciences is a Clinical CRO offering product development, trial, and drug safety management. Includes information on services, company background, careers, and locations
The company generated revenue of USD2.259 billion, an increase of nearly 24.73% from the previous year. The company provides clinical trial expertise using a clinical development platform that includes approximately 70 offices across North America, Europe, Asia, Latin America, South Africa, Australia, and the Middle East and over 15,000 employees worldwide.
Pharmaceutical Product Development (PPD) has generated revenue of USD 1.900 billion, is a privately held organization with a strong portfolio of integrated drug development, laboratory, and lifecycle management services including clinical, pre-clinical, post-clinical, and commercialization services. The company has 89 offices in 47 countries with more than 20,000 employees.
Charles River provides products and services to help expedite the discovery, early-stage development, and safe manufacture of novel drugs and therapeutics. They have generated revenue of USD 1.857 billion, an increase of nearly 10.47% from the previous year. The company had approximately 11,800 employees as of December 30, 2017.
ICON is a global provider of outsourced development services to the pharmaceutical, biotechnology, and medical device industries. They generated revenue of USD 1.758 billion, an increase of nearly 5.52% from the previous year. The company specializes in the strategic development, management, and analysis of programs that support all stages of the clinical development process – from compound selection to Phase I-IV clinical studies. ICON currently, operates from 98 locations in 38 countries and has approximately 13,250 employees.
Wuxi AppTec is a Chinese company that is a contract researcher for most of the largest pharmaceutical, biotech, and medical device companies and many smaller companies.
Wuxi AppTec provides a broad and integrated portfolio of laboratory and manufacturing services throughout the R&D process.
The Services include: A full range of discovery and development services for pharmaceuticals; development and testing services for biotherapeutics and medical devices; and comprehensive toxicology services as well as manufacturing services for advanced intermediates and active pharmaceutical ingredients (APIs); cell banking services; and cGMP manufacturing for cellular therapeutics and combination/tissue-based products
Wuxi Apptec has generated revenue of USD 1.011 billion, an increase of nearly 10% from the previous year. The company’s platform is enabling almost 3,000 innovative collaborators from more than 30 countries to bring innovative healthcare products. WuXi AppTec has 14000 employees.
Medpace is a clinical research organization (CRO) conducting global clinical research for the development of drugs and medical devices. They are Headquartered in Cincinnati, Ohio, Medpace Holdings, Inc has generated revenue of USD 0.436 billion, an increase of nearly 3.56% from the previous year. The company is a scientifically driven, global, full-service clinical contract research organization (CRO) providing Phase I-IV clinical development services. The company has therapeutic expertise across all major areas including oncology, cardiology, metabolic disease, endocrinology, central nervous system, and anti-viral and anti-infective. Medpace employs approximately 2,500 people across 35 countries.
CROs
Source: Explore Biotech

Add a Comment